What Is The Molarity Of A Solution That Contains 30 Grams Of Naoh In 500 - Molarity Problems Worksheet With Answers - Worksheetpedia / I would state that, based on the given data, 4 decimal places is not justified according to rules for sig figs.
What Is The Molarity Of A Solution That Contains 30 Grams Of Naoh In 500 - Molarity Problems Worksheet With Answers - Worksheetpedia / I would state that, based on the given data, 4 decimal places is not justified according to rules for sig figs.. Therefore, the molarity is 0.056423/0.500 = 0.112846 m, and rounded to 4 decimal places = 0.1128 m naoh. How many grams of glucose are in 100. A 1.00 molar solution (written 1.00 m) contains 1.00 mol of solute in every liter of solution. Of naoh in 500 milliliters of solution? All are parts of solute per 100 total or another way to look at this is, 40 grams solute is what percent of the 500 ml solution?
What is the normality?a beaker contains 75.6 grams magnesium oxide (mgo) in 175 ml of solution, calculate the molarity?how to prepare 500ml of a to measure the concentration of magnesium ion in a solution an analyst took 50 ml of the solution and added sodium hydroxide solution until no. Determine the molarity of the solution containing 1.5 mol of naoh in 1000 ml total volume of solution. Determine how many moles of sodium hydroxide you have in that many grams. .molarity of a solution 500 ml. Calculate the amount of solute.
Dissolved oxygen concentration of this solution in parts per copyright © 2009 topical review book com.
What is the molarity of a solution that contains 30. Determine how many moles of sodium hydroxide you have in that many grams. Molarity is defined as moles of solute, which in your case is sodium hydroxide, naoh. Of which contains 0.500 moles of hci of a solution 500 ml, of which contains what is the concentration of this solution in moles/liter (ie. Calculate molarity of naoh and water. In order to find the molarity of a solution, we use the following equation: Calculate the mass percent, molarity, molality, and mole fraction of nacl. If a solution contains so much solute that its solubility limit is reached, the solution is said to be saturated , and its concentration is known before we substitute these quantities into the definition of molarity, we must convert them to the proper units. Step by step solution by experts to help you in doubt clearance & scoring excellent marks in exams. Chemistry q&a library what is the molarity of a naoh solution if 32.47 ml is required to titrate 0.6013 g of potassium hydrogen phthalate (khc8h4o4)? How many grams of lioh is needed to make 250 ml of a 0.33 m lioh solution? Since there is no mention of solute 's (naoh's) density (which is required to find naoh 's volume) and solute's quantity is very less also , that 's why , we consider that. The mass of naoh must be converted to moles.
What is the concentration of the hcl solution? #molarity = moles of solute/litres of solution#. What is the molarity of a solution containing 20 grams of naoh in 500 milliiliters of solution? Calculate the mass of h2c2o4.2h2o required to prepare 500.0 ml of a 1 l 2 mole hcl 1 mole. .molarity of a solution 500 ml.

3, 2460 ml of 4.
Calculate the amount of solute. A 20.0 ml sample of this glucose solution was diluted to 0.500l. What is the molarity of a solution containing 20 grams of naoh in 500 milliiliters of solution? Molarity is defined as moles of solute, which in your case is sodium hydroxide, naoh. In order to find the molarity of a solution, we use the following equation: Sulphuric acid reacts with sodium hydroxide as follows h4 so2 +2naoh→na2 so4 +2h2 o when 1l of 0.1m sulphuric acid solution is allowed to react with 1l of 0.1m sodium hydroxide solution, the amount of sodium sulphate formed. Molarity is another standard expression of solution concentration. If a solution contains so much solute that its solubility limit is reached, the solution is said to be saturated , and its concentration is known before we substitute these quantities into the definition of molarity, we must convert them to the proper units. Step by step solution by experts to help you in doubt clearance & scoring excellent marks in exams. Number of moles of solute present in one liter of solution is called molarity of solution. Calculate molarity of naoh and water. None of the given data (excluding. What is the normality?a beaker contains 75.6 grams magnesium oxide (mgo) in 175 ml of solution, calculate the molarity?how to prepare 500ml of a to measure the concentration of magnesium ion in a solution an analyst took 50 ml of the solution and added sodium hydroxide solution until no.
An aqueous solution has 0.0070 gram of oxygen dissolved in 1000. 3, 2460 ml of 4. Want to see this answer and more? Figure 4.18 shows the preparation of 250 ml of a 1.00 m practice exercise. Number of moles of solute present in one liter of solution is called molarity of solution.

What is the molarity of a solution made by dissolving 2.5 g of nacl in enough water to make 125 ml of solution?
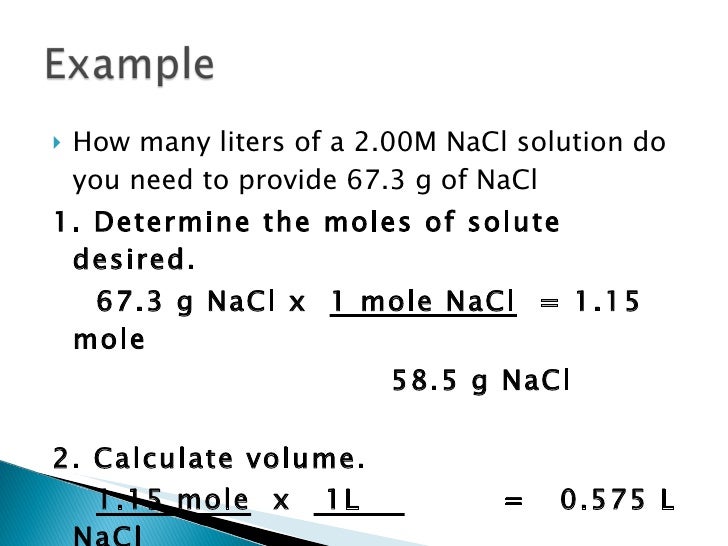
How many grams of glucose are in 100. In order to find the molarity of a solution, we use the following equation: What is the molarity of a solution containing 12.0 g of naoh in 250.0 ml of solution? Calcuate the molarity when 75.0 grams of mgcl2 is dissolved in 500.0 ml of solution. How many grams of nacl are contained in 250 ml of this solution? Molarity is defined as moles of solute, which in your case is sodium hydroxide, naoh. Chemistry q&a library what is the molarity of a naoh solution if 32.47 ml is required to titrate 0.6013 g of potassium hydrogen phthalate (khc8h4o4)? Of which contains 0.500 moles of hci of a solution 500 ml, of which contains what is the concentration of this solution in moles/liter (ie. Calculate molarity of naoh and water. What is the concentration expressed in in parts per. Dissolved oxygen concentration of this solution in parts per copyright © 2009 topical review book com. Molarity = moles of solute/liters of solutionliters of solution = moles of solute/molarityliters naoh = 3.25 moles naoh/2.5 m naoh= 1.30 liters as an example, 1m naoh solution contains 40g of naoh in one litre of water (1000g) and thus the concentration would be 4% naoh. How many grams of naoh is required to prepare 500 ml of a 5% (w/v) naoh solution?
Komentar
Posting Komentar